What are the chemical properties of the attractants?

NB. When handled properly, the attractants are all safe to use. However, all are potentially toxic and/or inflammable when not used with due precautions. For safety information, click here.
Carbon Dioxide CO2; molecular weight 44
Carbon dioxide is a colourless gas. At normal pressure it forms a solid on cooling rather than a liquid, melting point 78.5°C.
It is the by-product of oxidative respiration in all living organisms, and hence many insects which feed on the blood of living animals are attracted by it (e.g. tsetse, mosquitoes).
However, carbon dioxide is rather non-specific and is also present in the atmosphere at approximately 0.03-0.04%. It is technically more difficult to detect small increases in the carbon dioxide concentration "signal" against this non-zero and fluctuating background "noise". It is thus surprising that tsetse can apparently detect the carbon dioxide produced by an ox at over 64 metres.
Safety: Carbon dioxide is available in cylinders as a by-product of brewing. It can also be obtained as the solid "dry ice" or produced chemically by e.g. the action of acid on calcium carbonate (e.g. chalk, limestone).
Carbon dioxide is an odourless, non-flammable gas. It is present in the atmosphere (0.03-0.04%) and in exhaled human breath (4%) and at such levels is non-toxic. At higher concentrations it can cause hyperventilation and asphyxiation.
ACETONE (C3H6O; molecular weight 58)
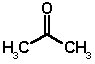
Acetone is a colourless liquid, boiling point 56°C, flash point -17°C, density 0.79. It is highly inflammable and miscible with both water and organic solvents such as chloroform.
Acetone is a by-product of lipid metabolism. AcetoacetylCoA is converted to free acetoacetate which is decarboxylated to acetone in the liver. Acetone in the blood can diffuse into the breath in the lungs. This is not the normal metabolic process, but acetone production increases under certain conditions such as starvation, diabetes and lactation.
Acetone is readily available from many chemical suppliers, being widely used as a solvent in chemical processes and products (e.g. nail varnish remover).
Safety: Acetone is a volatile (bp 56°C), highly inflammable liquid. It must be stored in strong, well-sealed containers, and care taken when opening these in case of pressure build up. Being so volatile, high concentrations of the vapour can easily occur and these can be suffocating as well as being easily ignited. Acetone is a good solvent, dissolving paint and attacking some plastics, and potentially penetrating the skin. It is miscible with water and organic solvents, and hence can be easily diluted with water in case of accidents. (MSDS)
BUTANONE (methyl ethyl ketone; C4H8O; molecular weight 72)
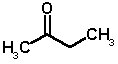
Butanone is a colourless liquid, boiling point 80°C, flash point -3°C, density 0.81. Like acetone, it is highly inflammable and miscible with both water and organic solvents.
It is not produced naturally by tsetse hosts. As a homologue of acetone it probably acts on the same odour receptors on the tsetse antennae.
Butanone is readily available and also widely used as a solvent. It can be used in place of acetone as an attractant - it may sometimes be cheaper, it is less volatile so easier to handle under hot conditions and it diffuses out of sealed polyethylene dispensers at a significant rate, unlike acetone.
Safety: Butanone has very similar properties to acetone except that it is less volatile (bp 80°C) and hence easier to handle and marginally less hazardous. Unlike acetone, butanone is only partially miscible with water. (MSDS)
OCTENOL (1-octen-3-ol; C8H16O; molecular weight 128)
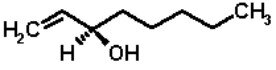
Octenol is a colourless liquid, boiling point 84-5°C/25mm, flash point 61°C, density 0.83. It has a strong smell of mushrooms, being a major component of mushroom volatiles. The molecule is "chiral", i.e. it exists in two mirror image forms. The octenol produced by cattle was shown to be an 80:20 mixture of (R) and (S) forms. The octenol from mushrooms is exclusively the (R) form.
Octenol is formed during oxidative breakdown of linoleic acid. Although it was originally assumed that octenol was produced in the breath of cattle, this has not been proven, and the exact origin is uncertain. However, octenol has been implicated in the chemical ecology of several other insect species, e.g. as an attractant for certain species of mosquitoes.
Octenol is available in small quantities from most chemical suppliers. Larger quantities are available from International Fragrances and Flavors.
Safety: Octenol (1-octen-3-ol) is a liquid with strong smell of mushrooms. It can be nauseating at high concentrations, but is otherwise relatively non-hazardous. (MSDS)
4-METHYLPHENOL (p-cresol; C7H8O; molecular weight 108)
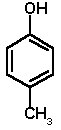
4-Methylphenol is a colourless solid, melting point 32-34°C, boiling point 202°C, flash point 89°C, density 1.03. Although when completely pure it is colourless, in practice it is generally more or less brown coloured due to the presence of small amounts of intensely-coloured quinone impurities formed by oxidation. These will also lower the melting point slightly so that the material may be liquid, depending upon the ambient temperature. 4-Methylphenol has a sharp odour, is a strong bacteriocide and is corrosive on skin or other tissues.
4-Methylphenol is generally the most abundant of the seven main phenols found in cattle urine. Like the other phenols, it is formed by degradation and hydroxylation of phenylalanine and tyrosine.
4-Methylphenol is readily available in bulk from most chemical suppliers, e.g. SigmaAldrich.
Safety: 4-Methylphenol (p-cresol) is a liquid on hot days and solid on cold days. It has a pungent, disinfectant-like smell, but the main hazard is that it is corrosive on skin and particularly damaging to sensitive tissues - e.g. it will cause severe burns to the digestive tract if consumed. Thus contact with skin should be avoided by handling with care and using gloves and protective clothing. It is significantly soluble in water, so that spills can be washed away with lots of water, particularly with soap or soda added. (MSDS)
3-METHYLPHENOL (m-cresol; C7H8O; molecular weight 108)
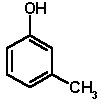
3-Methylphenol is a colourless liquid, melting point 8-10°C, boiling point 203°C, flash point 86°C, density 1.03. As with 4-methylphenol, it is usually more or less brown due to the presence of small amounts of quinone impurities. It also has a sharp odour and is corrosive to skin and other tissues.
3-Methylphenol is one of the seven phenols found in cattle urine, formed by metabolism of aromatic amino acids phenylalanine and tyrosine.
3-Methylphenol is available in bulk from most chemical suppliers, e.g. SigmaAldrich.
Safety: 3-Methylphenol (m-cresol) is a liquid with similar hazardous properties to those of 4-methylphenol. (MSDS)
3-PROPYLPHENOL (C9H12O; molecular weight 136)
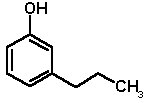
3-Propylphenol is a colourless liquid, boiling point 232°C, flash point 106°C, density 0.98. Material is usually more or less brown due to the presence of small amounts of quinone impurities. It has a slightly sharp odour and is corrosive to skin and other tissues.
3-Propylphenol is one of the seven phenols found in cattle urine, formed by metabolism of aromatic amino acids phenylalanine and tyrosine.
3-Propylphenol is not generally available, and cannot be substituted by 4-propylphenol or 3-isopropylphenol which are more widely available. It can be obtained from Great Lakes Fine Chemicals (UK) (formerly Palmer Research) or Appropriate Applications.
Safety: 3-Propylphenol is a liquid with similar hazardous properties to those of 4-methylphenol. (MSDS)